FDA Clears First Rapid At-Home COVID Test
The US Food and Drug Administration (FDA) issued an emergency use authorization for the first at-home COVID-19 diagnostic test, which provides results in 30 minutes or less.
The COVID-19 prescription All-In-One Test Kit (Lucira Health) is a molecular, single-use test that detects SARS-CoV-2 using self-collected nasal swab samples in people aged 14 years and older who are suspected of having COVID-19 by their healthcare provider.
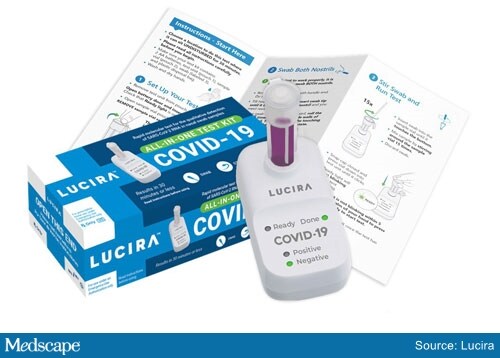
Lucira Health’s All-In-One Test Kit
The test is also authorized for use in doctor’s offices, hospitals, urgent care centers, and emergency departments for patients of all ages. However, samples must be collected by a healthcare provider when the test is used in these care settings for patients younger than 14 years. The Lucira test is currently available by prescription only.
“This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission. [This] action underscores the FDA’s ongoing commitment to expand access to COVID-19 testing,” said Hahn.
Rapid and Highly Accurate
The test kit includes the test device, sample vial, swab, and simple instructions. Two AA batteries are inserted in the device, and the sample vial is placed in the test unit.
In a community sample, in which the test was compared with an FDA-approved, high-sensitivity SARS-CoV-2 test, the Lucira test achieved a 94% positive percent agreement and a 98% negative percent agreement. After exclusion of samples with very low levels of virus that possibly no longer reflected active infection, Lucira’s test achieved 100% positive percent agreement, according to the company’s website.
The FDA said individuals with positive results should self-isolate and seek additional care from their healthcare provider. Those who test negative but who experience COVID-like symptoms should follow up with their healthcare provider, inasmuch as negative results do not preclude a person’s being infected with SARS-CoV-2.
While Lucira Health scales up manufacturing capabilities, its COVID-19 test kit will initially be available on a limited basis in point-of-care settings and healthcare networks that prescribe the test for patients to use at home. The company anticipates that the test will cost around $50.